TGA Compliance in the Body Contouring Industry : Are You Confused?
- Tanya White
- Sep 27, 2024
- 6 min read
Updated: Mar 15, 2025
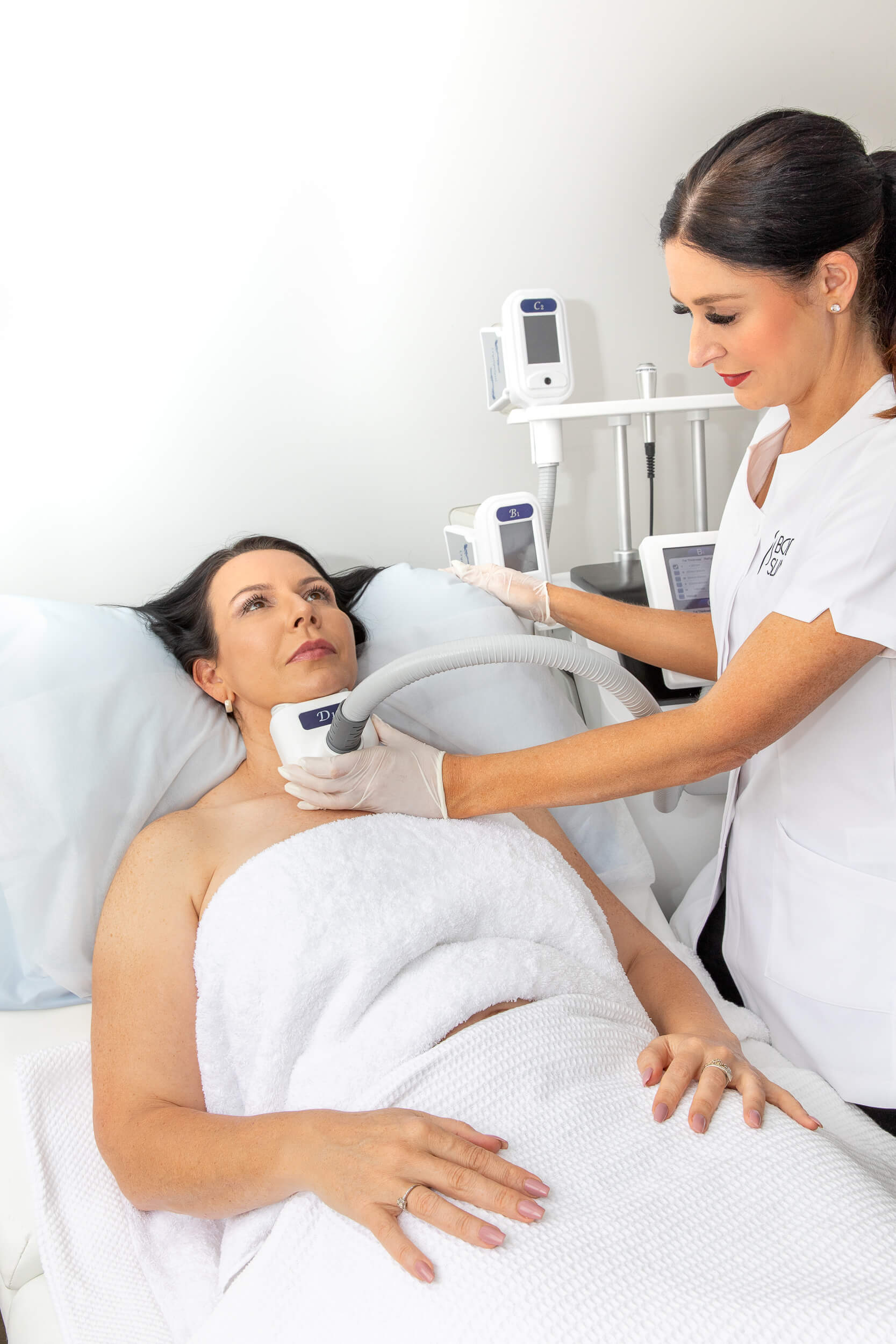
Are you finding yourself lost in a sea of confusing information about TGA compliance in the body contouring world? You’re not alone! Many salon owners and body contouring professionals are unsure about what’s actually required when it comes to the Therapeutic Goods Administration (TGA) regulations. But don’t worry—this guide is here to clear things up in a fun, engaging way. Let’s dive into the essentials of staying compliant, safe, and on top of your game!

What Is the TGA, and Why Should You Care?
The Therapeutic Goods Administration (TGA) is Australia’s regulatory body responsible for ensuring that therapeutic goods, including medical devices, meet specific standards for safety, quality, and effectiveness. In simple terms, the TGA keeps an eye on the tools and devices we use to make sure they are safe for the public.
As a body contouring professional, you need to know whether the machines you use in your practice are regulated by the TGA. Why? Because staying compliant protects your clients’ safety and your business!
What Exactly Are Body Contouring Machines?
Body contouring machines are those fabulous tools that help your clients achieve their desired body shape without surgery. These machines use a variety of technologies such as ultrasound, radiofrequency, lasers, fat freezing (cryolipolysis), and muscle stimulation to target fat reduction, cellulite smoothing, skin tightening, and muscle toning.
But not every body contouring device is regulated the same way under TGA rules. While some devices are likely to need TGA approval due to their claims, others might only need regulation depending on how you market them.
Fat Freezing and Muscle Stimulation: More Likely to Need TGA Approval
Let’s start with fat freezing (cryolipolysis) and muscle stimulation machines—two of the more powerful and popular treatments in body contouring. These devices are typically marketed with claims of permanent fat reduction or muscle enhancement, both of which are therapeutic claims that can affect the body’s structure and function. Because of these specific claims, they’re more likely to require TGA approval.
Fat Freezing (Cryolipolysis)
Fat freezing machines, like those used for cryolipolysis, are designed to freeze and break down fat cells, which the body then eliminates over time. If you’re marketing this treatment as one that reduces fat permanently, it’s likely to need TGA regulation. Since it’s altering fat tissues and has medical implications, the TGA considers these machines as higher risk, meaning they’ll fall under Class IIb medical devices.
Muscle Stimulation
Similarly, muscle stimulation devices, which are often used to build or tone muscles by stimulating them with electric pulses, are typically marketed with therapeutic claims like improving muscle tone or enhancing physical performance. These claims affect body function, making muscle stimulation devices subject to TGA approval, also falling under Class IIa or IIb medical devices.
Laser Lipolysis, Fat Cavitation, and RF Skin Tightening: It’s All in the Marketing
Now let’s talk about the devices that are on the cusp—those that may or may not require TGA approval, depending on how you market them. These include:
✨️Laser Lipolysis
✨️Fat Cavitation
✨️Radiofrequency Skin Tightening
The TGA's primary concern with these devices is how you position them in the market. If you’re marketing these machines as offering therapeutic outcomes like long-term fat reduction, cellulite treatment, or skin lifting, you’ll need to comply with TGA regulations. However, if you keep the marketing claims focused on cosmetic enhancements—like improving the appearance of the skin or temporary fat reduction without permanent change—they might not require TGA approval.
How Marketing Affects TGA Compliance

The TGA regulates devices based on their intended use and the claims you make about them. Here’s the general rule:
Therapeutic Claims (TGA-Approved): If you claim that your device treats or cures a medical issue (e.g., permanent fat reduction, skin tightening with medical effects), you’ll likely need TGA approval.
Cosmetic Claims (TGA Not Required): If you’re marketing your machine to improve appearance without making any therapeutic claims, it may not require regulation.
For example:
Fat Cavitation: Marketed for improving the appearance of fat? You’re probably safe without TGA approval. But claim it treats a condition or offers long-term results? That’s where TGA comes in.
Laser Lipolysis: If it’s marketed as body sculpting for cosmetic improvement, it may not need approval. But if the focus shifts to permanent fat reduction, TGA regulation may be required.
Radiofrequency (RF) Skin Tightening: It’s the same story here—focus on general skin improvement, and you may avoid TGA oversight. Claim medical benefits like long-term skin lifting, and regulation is likely needed.
Understanding TGA’s Device Classes
Here’s how the TGA classifies devices based on risk:
Class I: Low-risk devices (like bandages and basic medical tools).
Class IIa: Moderate-risk devices (including ultrasound machines and some body contouring technologies).
Class IIb Higher-risk devices (like fat freezing and muscle stimulation machines).
Class III: High-risk devices (like pacemakers, far removed from salon treatments).
Most body contouring devices you'll use fall into the Class IIa or IIb categories, meaning they may need TGA approval, particularly if marketed for medical outcomes.
How the TGA Regulates Your Body Contouring Machines
If your body contouring machine is categorised as Class IIa or IIb, it will need to go through a process called conformity assessment. This means the manufacturer needs to show the TGA that the device meets all safety, design, and performance standards. Once it passes, the device will be listed on the Australian Register of Therapeutic Goods (ARTG), which means it’s safe and effective for use in Australia.
The TGA will continue to monitor the device to ensure it remains safe, and manufacturers must report any safety issues or adverse events.
Why TGA Compliance Matters for You?
Why does all this matter to you as a body contouring professional? Simple. Using TGA-approved devices means you’re providing a safe, reliable, and effective service to your clients. It also means you're protecting yourself from potential legal risks.
Before you invest in any new machine, ask yourself these questions:
1. Is the machine listed on the ARTG?
2. Does the manufacturer provide proof of TGA compliance?
3. Does the device meet the safety standards required for professional use?
By doing your due diligence, you can feel confident that you're offering high-quality services while staying fully compliant with Australian regulations.
Disclaimer: This information is intended as a general guide only and should not be considered legal, regulatory, or professional advice. TGA compliance and requirements may vary based on individual circumstances, and it is the responsibility of each salon owner to ensure their claims, machines, and practices meet all applicable regulations. For specific guidance, please consult the TGA, a legal advisor, or your machine supplier.
💡 Did You Know?
You can join our exclusive SAFE SPACE Facebook group where we break down TGA compliance in simple, easy-to-follow terms and keep you updated on any changes in the body contouring industry. You’ll also get access to insider tips, free resources, and real-time advice on all things body sculpting!
Click here to join now and stay ahead of the game!
About Tanya White- The Author:

Tanya White is the proud owner of Australia’s number one body contouring training academy, Bodi Slim. With over 15 years of experience in the beauty industry, she has trained more than 500 students across Australia.
Tanya is passionate about helping beauty professionals thrive by providing expert training and mentorship in body contouring techniques. Her dedication to sharing knowledge and supporting her students ensures that every client receives safe, high-quality treatments. Ready to take your body contouring skills to the next level? Visit Bodi Slim for more information.
Want more expert tips and tricks on Body Contouring Salon Start-Up?
Meet Tanya White, the author of this blog and a Nationally Accredited body contouring trainer with over 15 years of experience in the beauty industry. Tanya runs Australia’s #1 online training academy, where she helps aspiring salon owners, beauty professionals, and entrepreneurs master the art of body contouring. She also offers personalised mentoring, certified training programs, and business support through her online courses.
Tanya is a nationally accredited trainer and has completed Training and Assessment (TAE40116). She has trained over 500+ students across Australia. Whether you’re looking to level up your skills with expert guidance or start your own body contouring business, Tanya’s got you covered!
Ready to Take Your Body Contouring Knowledge to the Next Level?
Visit Bodi Slim for more information
........... Oh and don't forget to join our
👉 What’s inside?
📚 FREE eBooks and Guides for Body Contouring Experts
📚 Access to free resources and training
🚀 Business strategies to scale your salon
🎥 Behind-the-scenes tips and transformation stories
🎓FREE Training Tips
Don't miss out on this amazing community—click here to join and connect with other Body Contouring pros today!



Comments